For US Healthcare Professionals Only
Delve into the science and HAE challenges



Examine the science of C1-INH to help inform treatment decisions
In 2019, Pharming partnered with a world authority on the pathogenesis and treatment of hereditary angioedema (HAE) to develop a visualization of the interconnectedness of the complement, contact activation, and fibrinolysis systems and how their interworkings lead to the symptomology experienced in HAE attacks.
Use this visualization to explore the role of C1 esterase inhibitor (C1-INH) in HAE attacks and potential HAE treatment challenges.
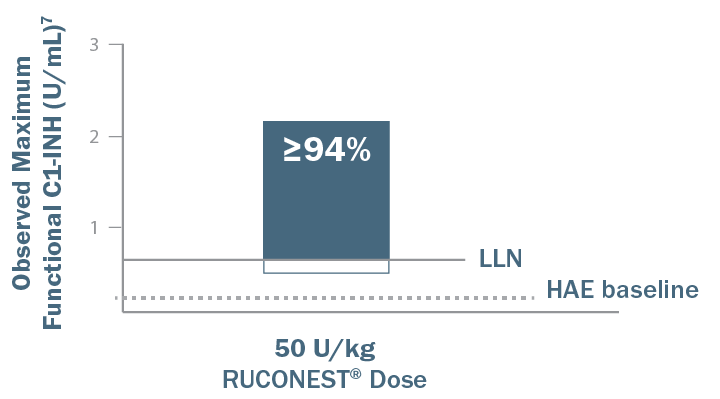
Redosing may prevent your patients from getting back to things that matter.

| * | According to a review of redosing and self-administration rates from the research literature.2 |
Redosing may prevent your patients from getting back to things that matter.

| * | According to a review of redosing and self-administration rates from the research literature.2 |
Gain insights into the experiences of patients with HAE.